Sodium nitrate is a white crystalline powder that is very soluble in water. Acids give carbon dioxide gas respective salt and water when they react with metal hydrogen carbonate.
When dissolved in water this compound actually produces a neutral solution.

Sodium nitrate acid or base. It exists as either trigonal or rhombohedral crystals. Remove the non-participating spectator ions from the ionic equation and balance the net ionic equation. Among the following salts which salts are acidic basic or neutral.
Sodium nitrate is salt of sodium hydroxide NaOH a strong base and Nitric Acid HNO3 strong acid thus on dissolving in water it gives a neural solution of pH 7 so it is neither an acid or base in water. Termokimia ΔfH0cair -452 kJmol ΔfH0padat -468 kJmol S0padat 117 JmolK Keamanan Ingesti Dapat menyebabkan gastroenteritis dan sakit perut. H 2 SO 4 2NaOH Na 2 SO 4 H 2 O.
Sodium nitrate can appear in a variety of physical forms from powdery or granular in consistency to a more solid rhombohedral crystal. This means that our bodies turn nitrate into nitrite. NaOH HNO 3 NaNO 3 H 2 O.
Sodium nitrate is a white crystalline solid with a density of 226 gmL and a melting point of 308 C. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. This food-grade additive has a melting point of 5864 degrees Fahrenheit 308 degrees Celsius.
Satuan SI digunakan bila mungkin. Tak seperti sebaliknya yang ditetapkan keadaan standar digunakan. H 3 PO 4.
Upon contact with saliva the chemical compound converts into sodium nitrite. Mata Dapat menyebabkan iritasi. Orthophosphoric acid Phosphoric acid Linear Formula.
NaNO3 commonly referred to as sodium nitrate is not an acid or a base. Nitrate naturally occurs in some vegetables fruits and grains. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
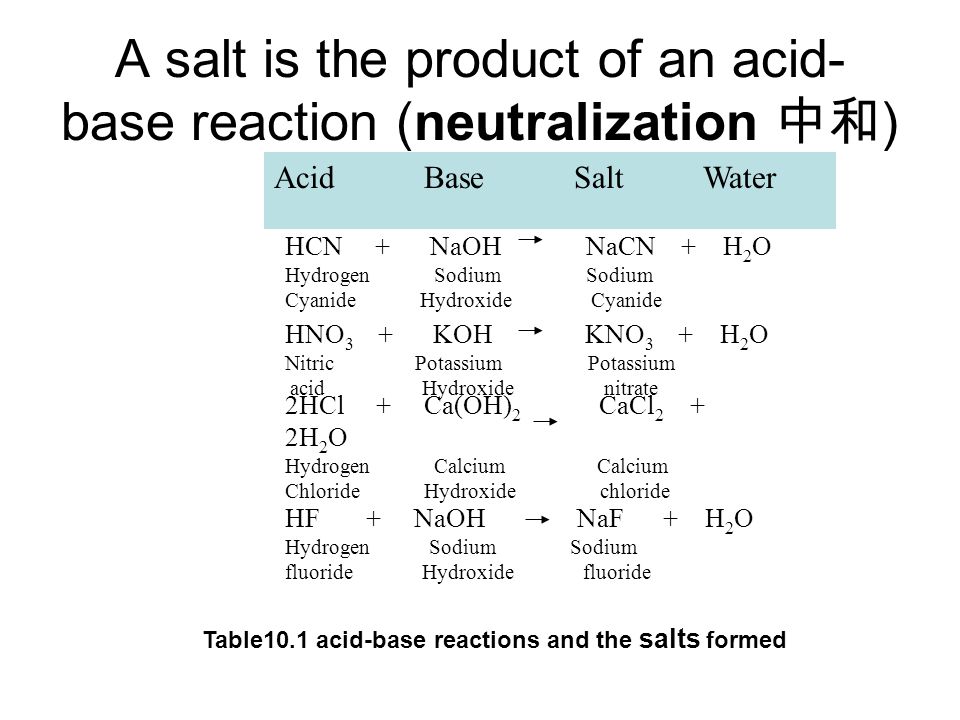
Nitric acid potassium hydroxide potassium nitrate water. SO 42-aq does not take part in the reaction because it is seen to be both a reactant and a product. Aluminium chloride zinc sulphate copper sulphate ammonium chloride.
Penghirupan iritasi pernapasan Kulit Dapat menyebabkan iritasi. Sulfuric acid sodium hydroxide sodium sulfate water. Natrium nitrat ialah tipe garam yang telah lama digunakan.
I Ammonium Chloride NH4Cl NH4OH HCl. It is a stable solid at room temperature however upon prolonged heating it can explode and release toxic. Acid Metal hydrogen carbonate Salt Carbon dioxide Water.
It is highly soluble in water and readily absorbs. Sodium nitrate is a neutral salt. Iii Sodium chloride NaCl NaOH HCl.
Is sodium nitrate an acid or base or neutral. Name of the salt Formula of salt Salt obtained from Base Salt obtained from Acid. Sodium chloride potassium nitrate aluminium chloride zinc sulphate sodium acetate sodium carbonate copper sulphate sodium sulphate ammonium chloride.
2H 2 O l. Sodium hydroxide nitric acid sodium nitrate water. Iii Reaction of acid with hydrogen carbonates bicarbonates.
HNO 3 KOH KNO 3 H 2 O. 2 K 2 OH- aq. 2 Haq SO42- aq.
Sodium nitrate NaNO3 is acid What is an acid base neutral. Iv Magnesium Nitrate Mg NO32 Mg OH2 HNO3. Hydrochloric acid magnesium hydroxide magnesium chloride water.
Sodium nitrate is the salt of a strong acid and hence dissociates completely in water into sodium and nitrate ions. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. Acids reacting with metal hydroxide bases.
Ii Copper sulphate CuSO4 Cu OH2 H2SO4. Nitric acid gives sodium nitrate water and carbon dioxide gas when it reacts with sodium carbonate.